The Particulate Nature of Matter
Kinetic Particle theory
When a solid is heated, particles vibrate faster about a fixed point causing particles to move further apart and so solid expands
When particles gain sufficient energy to overcome strong forces of attraction, they move out of their fixed position and can slide over each other in a continuous random motion -- solid has melted.
Particles in liquid have energy to move around but are still close to each other and do not have enough energy to overcome the forces that hold them close to each other.
If more heat is supplied, particles move faster until they have enough energy to overcome the forces of attraction. Particles escape the liquids surface and move around in continuous rapid motion -- the liquid has boiled
In the vapor, the particles move in rapid random motion. This movement is due to collision of vapor particles with air particles.
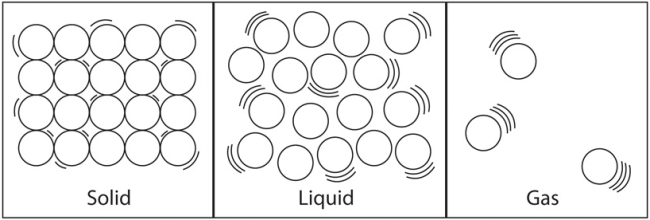
Solids, liquids and gases have different physical properties. The difference in these properties comes from differences in how the particles are arranged in each state.
States of Matter
| Solid | Liquid | Gas |
|---|---|---|
| Strong forces of attraction between particles | Weaker attractive forces than solids | Almost no intermolecular forces |
| Fixed pattern (lattice) | No fixed pattern, liquids take up the shape of their container | Particles far apart, and move quickly |
| Atoms vibrate but can't change position ∴∴ fixed volume and shape | Particles slide past each other. | Collide with each other and bounce in all directions |
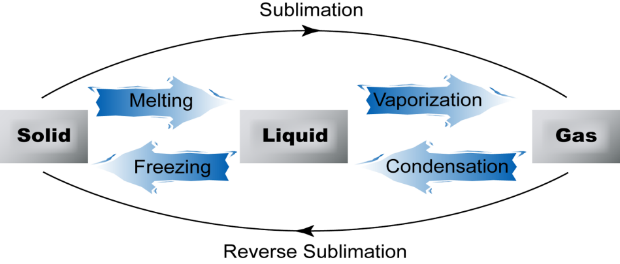
| Process | Heat Energy | Exo/endothermic |
|---|---|---|
| Melting | Gained | Endothermic |
| Boiling | Gained | Endothermic |
| Condensing | Lost | Exothermic |
| Freezing | Lost | Exothermic |
| Sublimation | Gained | Endothermic |
| Reverse sublimation | Lost | Exothermic |
Questions on the particle theory of matter show interconversion of states with a reversible arrow: ⇌, which means that the process can go forwards and backwards.
Read the question carefully and pick the direction of the change in state that the question refers to.
Heating Curve
Brownian motion
- Brownian motion is defined as the random movement of particles in a liquid or a gas produced by large numbers of collisions with smaller, often invisible particles
- The observation of Brownian motion proves the correctness of the kinetic particle theory
Diffusion
Diffusion is the spreading of one substance through another from a region of high concentration to a region of low concentration due to the continuous random motion of particles.
Evidence for diffusion:
In liquids: potassium manganate (VII) in a beaker of water
In gases: a gas jar of air and a gas jar of bromine connected
Factors that affect the rate of diffusion:
Temperature increases → rate of diffusion increases
Lower density gas → rate of diffusion is higher


Changes in State & Kinetic Theory
- When substances are heated, the particles absorb thermal energy which is converted into kinetic energy. This is the basis of the kinetic theory of matter
- Heating a solid causes its particles to vibrate more and as the temperature increases, they vibrate so much that the solid expands until the structure breaks and the solid melts
- On further heating, the now liquid substance expands more and some particles at the surface gain sufficient energy to overcome the intermolecular forces and evaporate
- When the b.p. temperature is reached, all the particles gain enough energy to escape and the liquids boils
- These changes in state can be shown on a graph which is called a heating curve
- Cooling down a gas has the reverse effect and this would be called a cooling curve
- These curves are used to show how changes in temperature affect changes of state
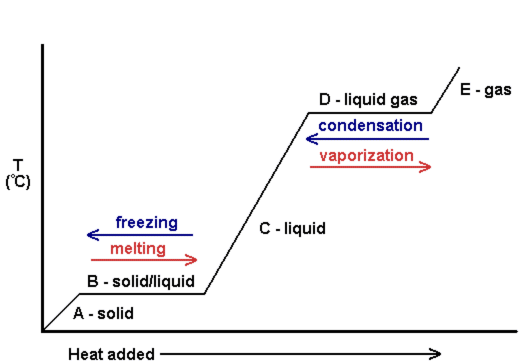
While changing state, the temperature of the substance remains the same as the heat energy is rapidly converted into kinetic energy. This is called latent heat and corresponds to the horizontal sections of a heating / cooling curve.