Electricity and Chemistry
Decomposition of an electrolyte with the help of electric current
Electrolyte:
Aq. solution of ionic substance or molten ionic salt
Conducts electricity due to the presence of mobile ions
Electrodes:
Rods which help current enter the electrolyte
Inert electrodes: do not take part in the reaction
Reactive electrodes: take part in the reaction
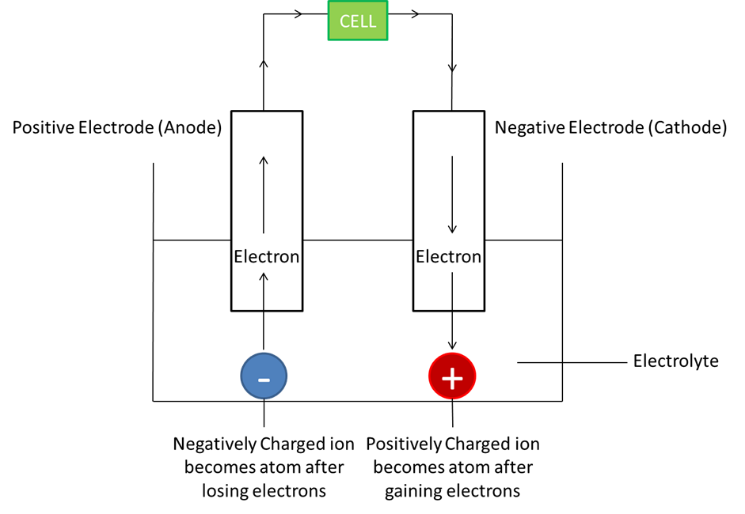
Principle
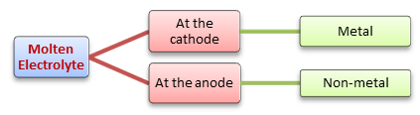
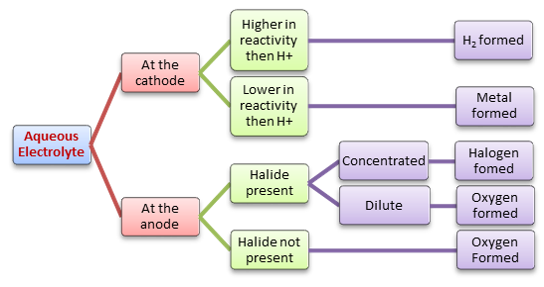
Examples
| Electrolyte | At cathode | At anode |
|---|---|---|
| Molten lead(II) bromide | Lead | Bromine |
| Concentrated hydrochloric acid | Hydrogen | Chlorine |
| Concentrated aqueous sodium chloride | Hydrogen | Chlorine |
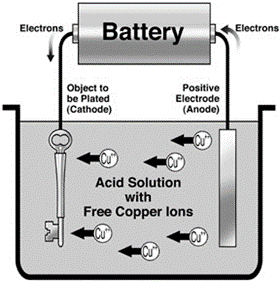
Electroplating
Coating one metallic object with another metal using electrolysis
For electroplating, you need:
Anode made of metal you want to electroplate object with
Ions of same metal as anode in solution
Object to be plated at cathode
Used to:
Make things look better
Prevent corrosion
Uses
Aluminium | Copper | Plastic & Ceramics |
|---|---|---|
Used for electricity cables because:
| Used in electrical wires as it is:
| Used as insulators because they:
|
Cables have steel core, for strength | Plastic used for casing in plugs | |
Ceramics used to support cables in electricity pylons |
Refining Metals
This is purifying impure metals
Rules:
Cathode: thin strip of pure metal
Anode: impure metal
Electrolyte: Aqueous Salt Solution of metal
Example:
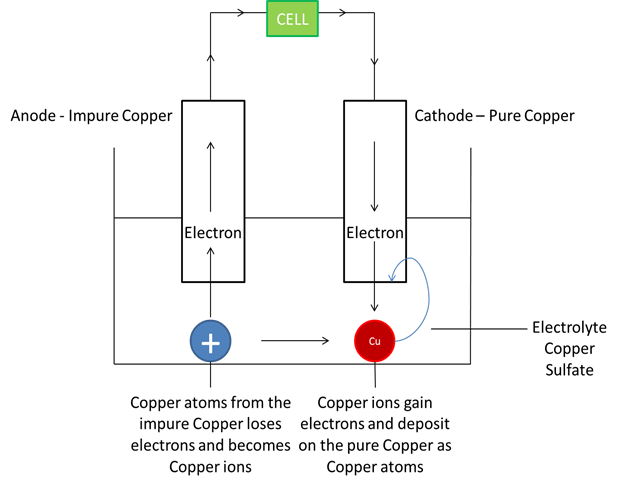
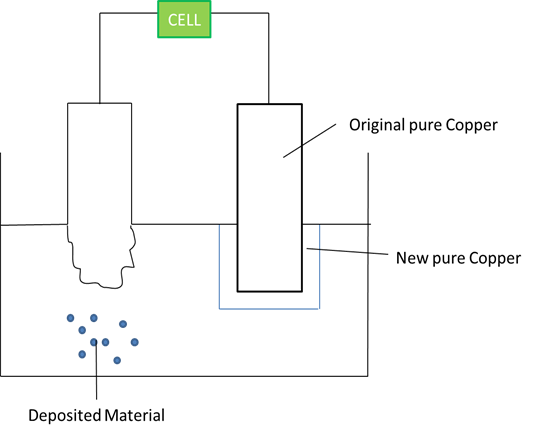
Reaction at Anode: Cu -- 2e Cu^2+^ (mass decreases)
Reaction at Cathode: Cu^2+^ + 2e Cu (mass increases)
Basics
Electrolysis is a way to decompose compounds, using electricity.
Reduction of positive cations happens at the cathode
Oxidation of negative anions happens at the anode
For example:
At the anode: 2Cl^-^ → Cl~2~ + 2e^-^
At the cathode: 2H^+^ + 2e^-^ → H~2~
Extraction of Aluminium
The main ore of aluminium is bauxite -- high m.p.
Aluminium (III) oxide (alumina) is dissolved in molten cryolite (Na~3~AlF~6~) -- this mixture has a lower m.p. (industrially preferred)
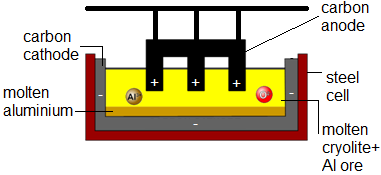
During electrolysis aluminium is produced at the carbon cathode and oxygen at the carbon anode.
Due to the high temp. the oxygen reacts with the graphite anode to form CO~2~ and so anode had to be periodically replaced
Electrolysis of Brine
Brine is concentrated NaCl solution
Ions present: Na^+^, H^+^, Cl^-^ and OH^-^
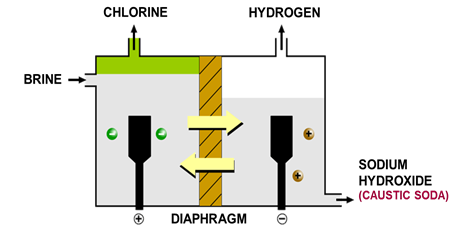
| At the anode | At the cathode |
|---|---|
| Made of titanium | Made of steel |
| Chlorine gas evolved | Hydrogen cations reduced to H~2~ molecules |
| Unreacted ions (Na^+^, H^+^ and OH^-^) move through porous membrane due to difference in liquid pressure | Left Na^+^ and OH^-^ which is aqueous sodium hydroxide |
| Net flow to the right |