Chemical Reactions
Collision Theory
- Collisions are needed for a chemical reaction to take place
- Successful collisions have enough activation energy at moment of impact to break preexisting bonds and form new bonds
Rates of Reaction
Rate of a chemical reaction is the concentration of reactant used up or product made in a given time.
Unit = (mol/dm^3^)/s
Concentration
Increasing concentration of reactants increases rate of reaction
This is because there are more particles per unit volume, so the collision rate between reacting particles increases, therefore the successful collision rate increases, which results in an increased rate of reaction.

Temperature
Increasing temperature increases the rate of reaction
This is because average kinetic energy of particles increase which means they are moving faster & also more particles have an energy greater/equal to activation energy, therefore successful collision rate increases, resulting in increased rate of reaction
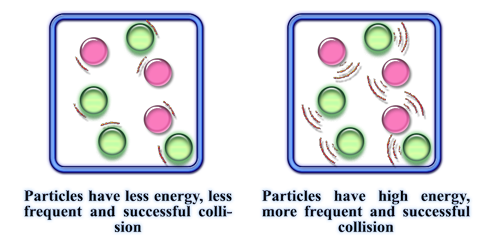
Particle Size
Decreasing the particle size (increasing surface area) increases the rate of reaction
This is because there are more reactant particles exposed to collide, so the collision rate increases, therefore the successful collision rate increases, resulting in an increased rate of reaction

- Large surface area can mean danger. For example, flour dust and wood dust have large surface areas, and are combustible. A spark from a machine, or a lit match, can cause an explosion. This also applies to gases from mines.
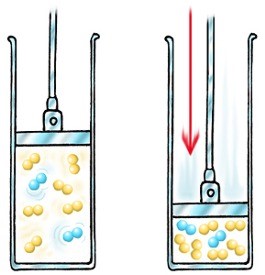
Pressure in Gaseous System
Increasing the pressure in a gaseous system increases the rate of reaction
The distance between particles is reduced under pressure
There are more particles per unit volume, so the collision rate increases, therefore the successful collision rate increases, resulting in an increased rate of reaction.
Catalyst
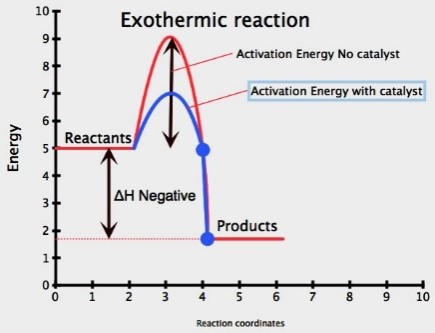
A catalyst is a substance (usually a transition metal) which speeds up a chemical reaction, but remains unchanged at the end
Adding a catalyst increases the rate of reaction
A catalyst allows the reaction to go by an alternative pathway with lower activation energy
More particles will have an energy greater than or equal to the activation energy, therefore successful collision rate increases resulting in increased rate of reaction
For gaseous reactants, if catalyst is solid metal, the catalyst provides a surface for reaction to take place on
The larger the surface are of the metal catalyst, the larger the area for reaction to take place therefore higher rate of reaction
Enzymes are protein molecules. They are biological catalysts which speed up reactions but remain chemically unchanged at the end
Enzymes function best at optimum temperature and pH level otherwise they may denature and completely stop functioning
Measuring Rates of Reaction Experimentally
| Gas Evolved | Mass Loss | Colour Change |
|---|---|---|
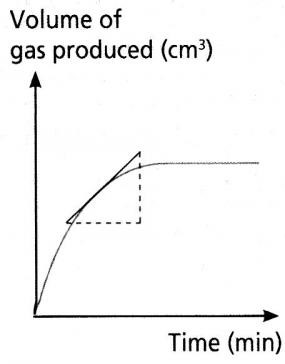 | 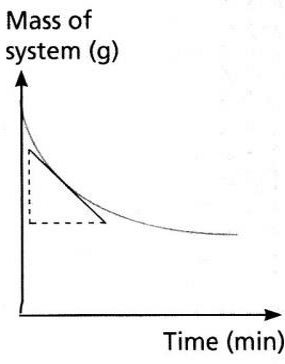 | 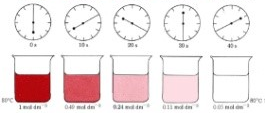 |
| If a gas evolves, measure volume of gas produced per unit time using a gas syringe | If a gas evolves, measure loss in mass per unit time by placing on a balance then putting a cotton wool on top to allow gas to pass but not to enter | If a colour change occurs we can measure the time taken to go cloudy |
Light Causing a Chemical Reaction
A photochemical reaction is one where light causes a reaction to occur. The higher the light intensity the higher the rate of the reaction.
Photosynthesis: light provides energy for the reaction and chlorophyll is a dye that absorbs light.
carbon dioxide + water → (light + chlorophyll) → glucose + oxygen
6CO~2~ + 6H~2~O → (light + chlorophyll) → C~6~H~12~O~6~ + 6O~2~
- Silver salts in photographic film: Silver bromide breaks down, where light strikes the film, so silver is reduced. Silver ions are reduced to silver.
2AgBr~(s)~ →2Ag~(s)~+Br~2(g)~
Reversible Reactions
A reversible reaction is a reaction in which reactants form products and the product(s) can then react or decompose to form the reactants
Example: CuSO~4~.5H2O (blue) ⇌ CuSO~4~(white) + H~2~O
(to get anhydrous, heat it, & to get hydrated form, add water)
There are two types of equilibrium: static and dynamic.
At dynamic equilibrium:
Rate of forward reaction = rate of reverse reaction
Concentrations of all reactants and products remain constant
System is closed, and on large scale everything is constant
Equilibrium
Le Châtelier's Principle: if conditions of an equilibrium are changed, the position of equilibrium moves to oppose change
Temperature: Temperature lowered; equilibrium moves in exothermic direction. Temperature raised; equilibrium moves in endothermic direction.
Pressure: Pressure raised; equilibrium moves to side with fewest gas molecules. Pressure lowered; equilibrium moves to side with most gas molecules.
Concentration: Decreasing reactant concentration or increasing product concentration; equilibrium moves to reactant side. Increasing reactant concentration or decreasing product concentration; equilibrium moves to product side.
Redox
- A redox reaction is one in which one species has been oxidized and another species has been reduced
| Oxidation means: | Reduction means: |
|---|---|
| Loss of electrons | Gain of electrons |
| Gain of oxygen | Loss of oxygen |
| Loss of hydrogen | Gain of hydrogen |
OIL RIG
Reducing agents are oxidized and oxidizing agents are reduced
Potassium iodide is a reducing agent & will go from colorless to red-brown, so is oxidized to produce I~2~
H~2~O~2~ + 2KI + H~2~SO~4~→I~2~ + K~2~SO~4~ + 2H~2~O
Potassium manganate is an oxidising agent and will go from purple to colourless
To test for an oxidising agent, add a reducing agent (KI) and to test for a reducing agent, add an oxidising agent (KMnO~4~)